PACKi TRACKnTRACE
Introduction
The Pharmaceutical industry is not only an important source of medical innovation, but also a heavily regulated industry. Serialization process is a dynamic aspect in it. Our proven robust technology meets all your serialization requirements, irrespective of your packaging lines. Our credentials and expertise have enabled us to emerge with total solutions for implementation for barcoding and serialization requirements.
Product Features
- Secured & reliable software design ensures minimal scope for human errors
- User-friendly multilingual user interface
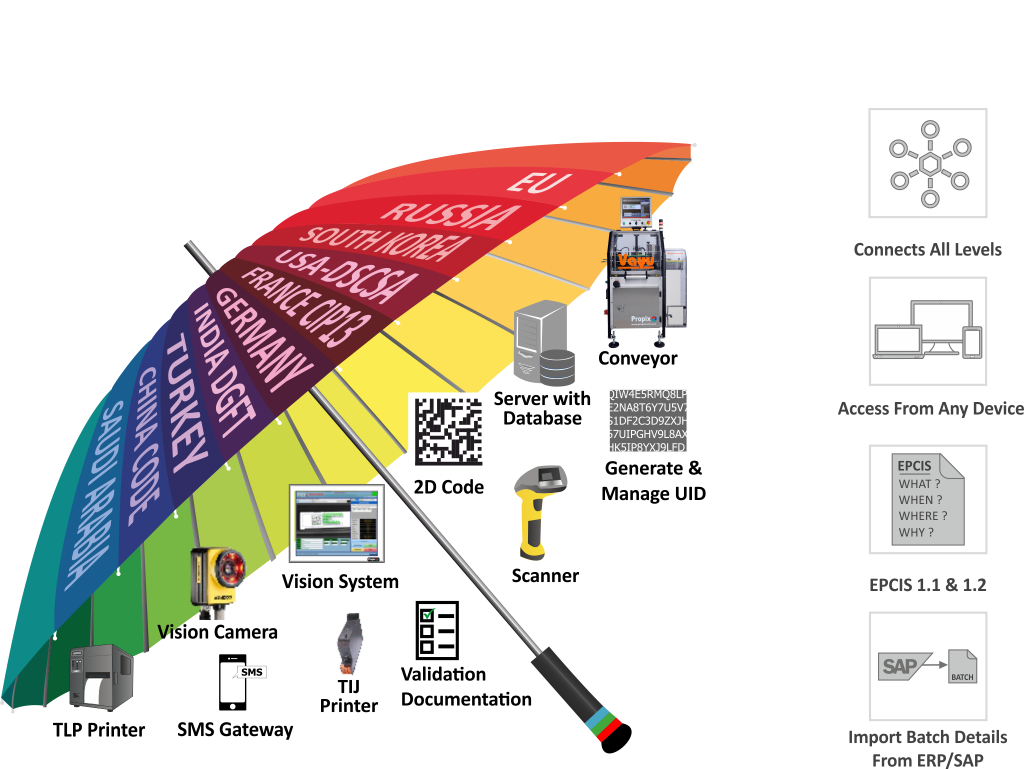
- Centralized software to meet serialization compliance of all countries across the globe.
- Ready to use third party integration like Tracekey, Rfxcel, Tracelink, Werum, SAP, Adents, Verify brand, De La Rue, Dentsu, Allexis, Russia MDLP, etc.
- Complete informative dashboard which displays real me status of all track and trace production lines and statistical data
- Optional ‘Connect+’ feature to connect with Track n Trace line (Level 2) of other make
- Generate unlimited UIDs without any additional cost
- Automatic selection of the respective UID provider
- Generates EPCIS 1.1 & 1.2 and .xml files as per country compliance as well as third party UID provider
- Facilitates product management, batch management & production line management
- Facilitates ‘Product detail’ import from ERP / SAP or external file
- Ease of line configuration and complete line management
- Line clearance feature gives flexibility to hold the currently running batch and run a new batch on the same line
- A unique feature to transfer batch from one line to another
- 4 user levels & role management for server & line level application
- Sync with active directory
- Various statutory MIS reports like audit trial, product / batch wise, user and reconciliation reports
- Password-protected PDF format reports
- Date-wise database backup & restore utility
- Label design utility for various label format design, no need of any external label design software
- Meets 21 CFR Part 11 compliance
For further information please see the flyer or contact us direct!